About
Perfluorosulfonic Acid Ion Exchange Membrane PSAIEM
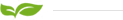
Perfluorosulfonic acid ion exchange membrane, also known as perfluorosulfonic acid proton exchange membrane and cation exchange membrane, is a solid polymer electrolyte, a strong acid type ion exchange membrane, with good chemical and thermal stability, low voltage, and low membrane resistance. High conductivity, good hydrophilicity, high water content of the membrane, high mechanical strength, etc., can be used under harsh conditions such as strong acid, strong alkali, strong oxidant medium and high temperature. Due to its own characteristics, perfluorosulfonic acid Proton exchange membranes are not only used as key components of proton exchange membrane fuel cells, but also widely used in the chlor-alkali industry, electrolysis,all-vanadium flow batteries, hydrogen production by water electrolysis, electrochemical synthesis, and gas sensors.