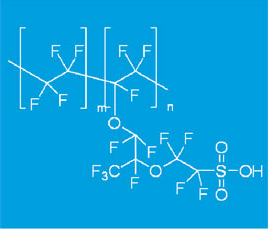
Chemical Name:PFSA Polymer Dispersions,Ion Exchange Resin Solution SY-1000
CAS Number:12627-85-9
Synonyms:PFSA Polymer Dispersions,Ion Exchange Resin Solution, PFSA Resin solution
Appearance:solution
Solution:5%,10%,15%,20%wt
Solvent:Alcohol or DMF
Application:for materials of ion exchange membrane
REF. FOB Price:USD150-500/L
Min. Order:10g
Payment Terms:L/C,T/T,Western Union,Paypal
Lead Time:10-15 days
Month Capacity:30000 liters
Adsorption selection:
Ion exchange resins have different affinities for different ions in solution and are selective for their adsorption. There are general rules for the strength of various ions exchanged and adsorbed by resin. The main rules are as follows:
1, adsorption of cations
High-valence ions are usually preferentially adsorbed, and among the same-valent ions, the ions with larger diameters are more strongly adsorbed. Some cations are adsorbed in the following order:
Fe3+> Al3+> Pb2+> Ca2+> Mg2+> K+> Na+> H+
2, the adsorption of anions
The general order of adsorption of inorganic acid radicals by strongly basic anion resins is:
SO42-> NO3-> Cl-> HCO3-> OH-
The general order of adsorption of anions by weakly basic anion resins is as follows:
OH-> citrate-> SO42-> tartrate-> oxalate-> PO43-> NO2-> Cl-> CH3COO-> HCO3-
3, Adsorption of colored substances
Sugar liquid decolorization often uses strong basic anion resin, which has strong adsorption to pseudo-melanin (reducing sugar and amino acid reaction product) and alkaline decomposition products of reducing sugar, while the adsorption of caramel color is weak. This is thought to be due to the fact that the first two are usually negatively charged, while caramel has a weak charge.
Generally, resins with a high degree of crosslinking are more selective for ions, and resins with macroporous structures are less selective than gel-type resins. This selectivity is larger in dilute solutions and smaller in concentrated solutions.
Properties of SY-1000 Ion Exchange Resin:
Properties |
Values |
Purity |
99.9% |
Acid Capacity(meq/g) |
1.00 |
Exchange Equivalent (g/eq) |
960--1000 |
Melting Temperature |
280 C |
Decomposition Temperature |
>350 C |
Specific Gravity(g/cm3) |
1.98 |
1, A base titration procedure measures the equivalents of sulfonic acid in the polymer, and uses the measurement to calculate the acid capacity or equivalent weight of the pure polymer.
2, Thermogravimetric Analysis was performed to test the decomposition temperature. The polymer were placed inside a furnace and heated from room temperature to 600C at a heating rate of 10C/min.
Application:
1. As the material of the PFSA ion exchange membrane
SY-1000 perfluoro sulfonic acid ion exchange resin is used as the materials of ion exchange membrane, the fuel cell membrane, the ion exchange membrane for vanadium battery, the ion membrane for a chlor-alkali industry, the hollow fiber membrane, the super acid catalyst used in organic synthesis, the super stable ion exchanger, and the like are reprepared in various electrolysis apparatuses.
2. Fuel Cell Applications: PFSA polymer dispersion and PFSA resin solution is a diluted, liquid form of the same chemical used for a PEM fuel cell membrane, PFSA polymer dispersion, and PFSA resin solution can drastically reduce the amount of platinum needed as a catalyst by exposing a larger fraction of the platinum to the hydrogen gas. Also, PFSA polymer dispersion and PFSA resin solution act as a binding agents to hold the platinum, membrane, and gas diffusion layer together.