About
Chlor-alkali membrane SY287
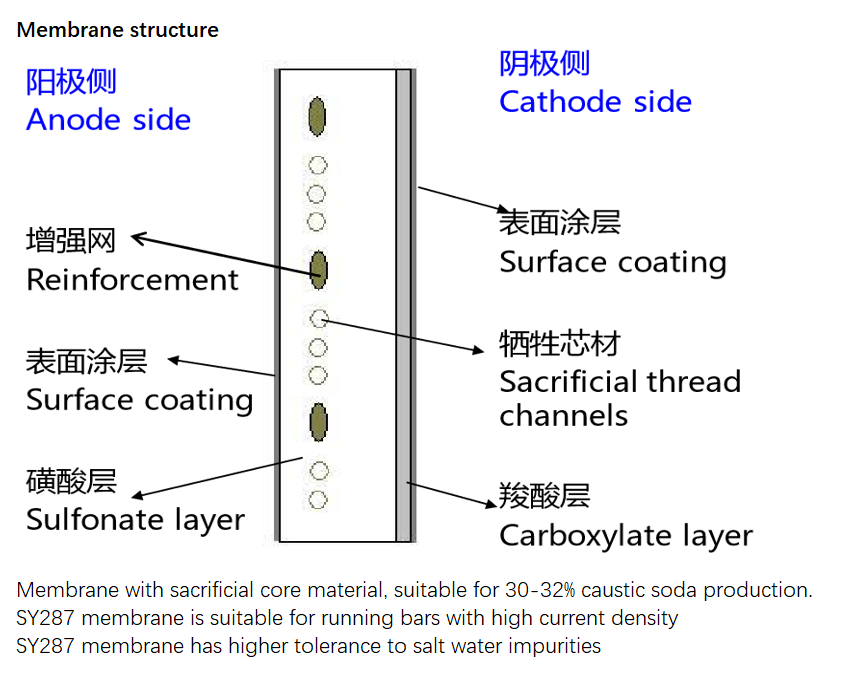
SY287 Ion-exchange membranes are reinforced membranes to improve the mechanical property of the composite membrane and allows the membrane to restrict its swelling. The membranes is suitable for 30-34% NaOH production, with sacrificial thread channels, equipped with the most advanced technological feature. Designed to work under higher current density with lower cell voltage and improved durability against brine impurities.
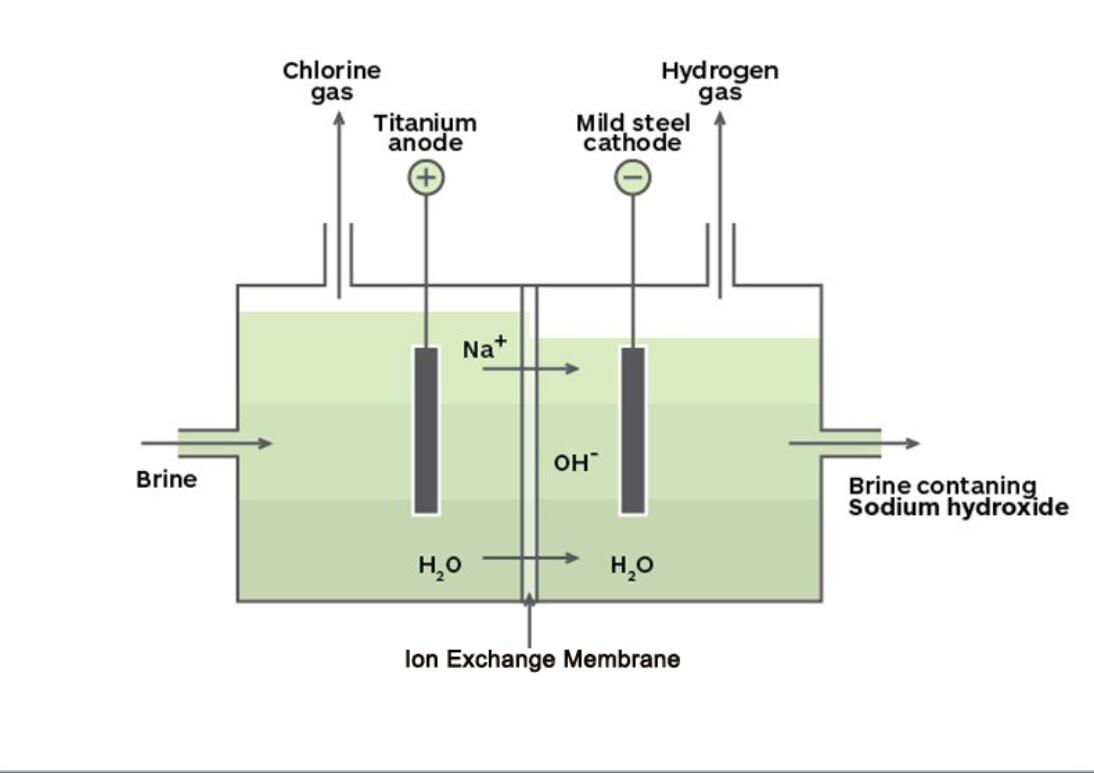
Chlor-alkali ion exchange membranes have selective permeability of cations. The membrane only allows cations such as Na + and water molecules to permeate, and other ions are difficult to permeate. During electrolysis, a strictly refined NaCl solution is injected into the anode compartment from the lower part of the electrolytic cell, and water is injected into the cathode compartment. After being energized, Cl-discharges in the anode chamber to generate chlorine gas, which is exported from the top pipeline of the electrolytic cell; at the same time, Na + carries a small amount of water molecules to the cathode chamber through the cation exchange membrane.
In the cathode chamber, H + discharges to generate H 2, which is led out from another pipeline on the top of the electrolytic cell. The remaining OH-is blocked by the cation exchange membrane and cannot move to the anode compartment. It is gradually enriched in the cathode chamber and forms a NaOH solution with the Na+ passing through the anode chamber.
As the electrolysis progresses, refined salt water is continuously injected into the anode chamber to supplement the consumption of NaCl; water is continuously injected into the cathode chamber to supplement the water consumption and adjust the concentration of the product NaOH. The resulting lye is led out from the upper part of the cathode chamber. Because the cation exchange membrane can prevent the passage of Cl -, the NaOH solution generated in the cathode compartment contains few NaCl impurities. The product produced by this method has a higher concentration, higher purity, and lower energy consumption than the product produced by diaphragm electrolysis. It is the most advanced chlor-alkali production process.